Undergraduate Student Report 2022
Teaching an old dog new tricks: Characterisation of the antifungal activity of toyocamycin against Candida albicans
Alessia Trombetta, Glasgow Caledonian University

Initially, we evaluated the antifungal activity of toyocamycin against planktonic and biofilm cells, this was done using conventional antimicrobial susceptibility testing and biofilm methods such as the crystal violet and XTT assays, which can quantify both the biomass and metabolic activity of biofilms. We then investigated any potential effects which toyocamycin has on the morphology of C. albicans cells within the biofilm using a live/dead fluorescent microscopy method. After our initial experiments, it was evident that toyocamycin was able to inhibit biofilm formation, however it has limited antifungal effect on biofilms which had been pre-formed. Given this finding, we next sought to investigate if there were any known biofilm tolerance mechanisms which were responsible for this limited effect. To potentially overcome this limitation, we investigated combination therapy of toyocamycin with two different compounds: DNase and an efflux pump inhibitor to see if the inhibition of these mechanisms could potentiate toyocamycin activity. We then quantified efflux pump activity in the presence and absence of toyocamycin, which enabled us to quantify the activity of these pumps in exporting toyocamycin following treatment. Finally, we performed an RNA extraction and cDNA synthesis before using a qPCR based method to quantify any changes in gene expression of three key genes involved in C. albicans biofilm formation: Als3, Hwp1 and Zap1.
Toyocamycin was very active against planktonic cells and also at inhibiting biofilm formation, with a concentration of 1μg/mL able to inhibit >80% of biofilm formation. However, when a biofilm had been pre-formed, this was able to tolerate increased concentrations of toyocamycin. The drug did not have any synergistic effect in combination with DNase and an efflux pump inhibitor, suggesting that a different tolerance mechanism(s) is involved in this process. Additionally, efflux pump expression in the presence of toyocamycin was not induced and sequential treatment over a 14-day period of constant toyocamycin therapy did not induce any resistance to the compound which was very promising. One of the most notable findings from the project was that toyocamycin was able to inhibit yeast-hyphae transition within biofilms (Fig1). This finding was supported by results from the qPCR data which reported decreased expression of Hwp1, with no changes reported in Als3 and Zap1. Findings are graphically summarised in figure 2.

Figure1. Fluorescent micrographs of C. albicans biofilms, untreated (A) and treated with 1μg/mL of toyocamycin (B). Note the differences in cellular morphology and lack of hyphae formation in the treated sample.
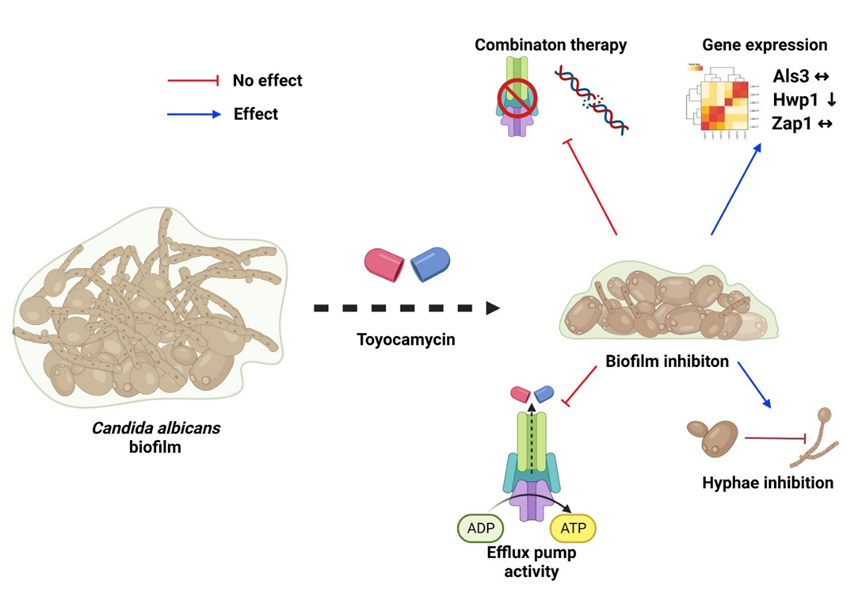
Figure 2. Proposed hypothesis of toyocamycin on inhibition of C. albicans biofilm. The figure illustrates the effects of the drug reported during the experiment. Toyocamycin had an effect on hyphae formation inhibition and gene expression, by decreasing Hwp1 gene expression. Additionally, it had no effect on inducing efflux pump activity and combination therapy with DNase and an efflux pump inhibitor.
This project gave me the opportunity to be a more confident scientist in the lab and increased my passion in pursuing post graduate research. During the eight weeks, it gave me invaluable experience from which I am sure I will benefit in my future after my undergraduate degree. This studentship has given me the opportunity to be able to develop my practical skills in the lab and look at all the different aspects related to working in research, it enhanced my problem solving and troubleshooting skills and made me appreciate that experiments do not always work first time. I also appreciated having the chance to interact and engage with undergraduate students undertaking other studentships, PhD and masters students, as well as other researchers and lecturers; having the chance to engage with them gave me the chance to discuss and understand different scientific careers. I am extremely grateful for these eight weeks in the lab, and for this I would like to thank the British Mycological Society and Dr Ryan Kean for giving me this opportunity, and contributing to my personal and academic development, as well as my future career.
Alessia Trombetta was supervised by Dr Ryan Kean, Glasgow Caledonian University.

